| CAS
NO. |
124-94-7
(Base), 76-25-5 (Acetonide) |
|
| EINECS
NO. |
200-948-7 |
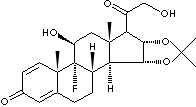
| FORMULA |
C24H31FO6 |
| MOL
WT. |
434.50 |
|
H.S.
CODE
|
|
|
TOXICITY
|
|
| SYNONYMS |
Triamcinolone 16a,17-acetonide;
|
|
9a-Fluoro-11b,21-dihydroxy-16a,17a-isopropylidene-dioxy-1,4-pregnadiene-3,20-dione;
9-Fluoro-11beta,16alpha,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
cyclic 16,17-acetal with acetone;
9alpha-Fluoro-11beta,16alpha,17,21-tetrahydroxypregna-
1,4-diene-3,20-dione cyclic
16,17-acetal with acetone;
|
|
SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
white to off-white
crystalline powder |
| MELTING POINT |
274 - 278 (Decomposes) |
| BOILING
POINT |
|
| SPECIFIC GRAVITY |
|
| SOLUBILITY
IN WATER |
Practically insoluble (soluble in alcohol, chloroform) |
| pH |
|
| VAPOR DENSITY |
|
|
REFRACTIVE
INDEX
|
|
|
NFPA
RATINGS
|
|
|
AUTOIGNITION
|
|
|
FLASH
POINT
|
|
| STABILITY |
Stable under normal conditions. |
|
GENERAL
DESCRIPTION & APPLICATIONS
|
Triamcinolone is a synthetic steroid of the glucocorticoid family. It is a mimic
of natural cortisol (hydrocortisone), acorticosteroid hormone produced by the
adrenal cortex. Triamcinolone has a fluorine atom in stead of hydrogen at 9
position. It is used clinically as an anti-inflammatory and immunomodulator in a
wide variety of conditions, including rashes, hemorrhoids, arthritis, and
inflammatory bowel disease. It is used in the replacement therapy for adrenal
insufficiency. Commercially available esters of triamcinolone include:
- Triamcinolone Benetonide [CAS
#: 31002-79-6]
- Triamcinolone Furetonide
[CAS #: 4989-94-0]
- Triamcinolone Hexacetonide [CAS
#: 5611-51-8]
- Triamcinolone Diacetate
[CAS #: 67-78-7]
- Triamcinolone Acetonide [CAS
#: 76-25-5]
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
white to off-white
crystalline powder |
|
IDENTIFICATION
|
Infrared
absorption (conforms to USP)
Ultraviolet absorption
(conforms to USP)
|
|
ASSAY
|
97.0
- 102.0%
|
|
HEAVY
METALS
|
20ppm
max
|
|
LOSS
ON DRYING
|
1.5%
max
|
|
SPECIFIC ROTATION |
+118°
~ +130°
|
| TRANSPORTATION |
| PACKING |
|
| HAZARD CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
|
Hazard
Symbols: XN, Risk Phrases: 40, Safety Phrases: 36/37-45 |
|
GENERAL
DESCRIPTION OF CORTICOSTEROID
|
Corticosteroid is a
synthetic or naturally occurring 21-carbon steroid
structure substances with four fused rings and vary functional branches attached to the ring
system. Cholesterol and steroid hormones have this carbon skeleton. The term steroid
refers to these three groups that contain a hydrogenated
cyclopentano perhydro phenanthrene ring system. In restricted context for medical
usage by non-endocrinologists, it refers to corticosteroids. Natural corticosteroids are elaborated by the adrenal
cortex in response to physiological carbohydrate metabolism. According to their
predominant biologic activity, they are divided into two major groups:
glucocorticoid and mineralocorticoid. Glucocorticoid's chief function is to
regulate carbohydrate, lipid, and protein metabolism and inhibit the release of
ACTH (adrenocorticotropic hormone). Glucocorticoids also affect muscle tone and
the microcirculation, participate in the maintenance of arterial blood pressure,
increase gastric secretion, alter connective tissue response to injury, impede
cartilage production, inhibit inflammatory, allergic, and immunologic responses,
invoke shrinkage of lymphatic tissue, reduce the number of circulating
lymphocytes, and affect the functions of the central nervous system. In humans,
the most important ones are cortisol, cortisone, and corticosterone.
Mineralocorticoids, uniuqely aldosterone in human, regulate the balance of water
and promote retention of sodium, loss of potassium. Aldosterone plays a role also
in promoting tissue repair. Spironolactone is the aldosterone antagonist called
potassium-sparing diuretic used in the treatment of hypertension.
Corticosteroids are used in clinically for hormonal replacement therapy, for
suppression of ACTH, as antineoplastic, antiallergic, and anti-inflammatory
agents, and to suppress immune responses.
- Glucocorticoid
Receptor Agonists
- Dexamethasone
- Triamcinolone
- Hydrocortisone
- Prednisone
- Glucocorticoid
Receptor Antagonists
- Mineralocorticoid
Receptor Agonists
- Mineralocorticoid
Receptor Antagonists
- Spironolactone
- Eplerenone
|
